Medical Physics & Oncology
December 2020 – July 2022: Artificial Intelligence for Oncology (LITO, Institut Curie, Orsay)
-
PANACEE (PANomic Atlas for non-small CEll lung cancer managEment) project: use of machine learning to identify a small group of patients with similar (radiomic and clinical) characteristics as patients (from a reference database) already treated for non-small cell lung cancer. The resulting tool (atlas + similarity search) will help doctors in finding the best therapeutic strategy to be adopted for the new subject projected into our database.
- PET/CT segmentation and radiomic features extraction (LIFEx software)
- Un-supervised clustering (dataset population represented as a graph which is clustered using modularity optimization)
- Search for twin patients (similar characteristics)
- Predictive models: cancer type and treatment response (breast and lung cancer)
October 2018 – October 2020: Mathematical modeling for Oncology (CRCM, SMARTC, Marseille)
- A*MIDEX project: Combining chemotherapy with immunotherapy is challenging due to a large choice of therapeutic molecules and administration protocols. Each molecule has an action towards a specific target that could be either the cancer cell, the endothelial cell, the regulatory T-lymphocyte or even the natural killer cell. The choice of the administration schedule of both molecules and their respective doses is not simple. The aim of this work is to implement a mathematical model that would combine chemotherapy and immunotherapy towards best efficacy (i.e. reducing tumor size) and lowest toxicity. We used the assumptions from several papers from the literature to develop a mathematical model that includes 3 populations of cells: Tumor cells, Cytotoxic T Cells (CTLs) and Regulatory T Cells (Tregs). The tumor induces a tolerant state in which the immune system can no longer respond to tumor antigens through direct inhibition of CTLs but also through the recruitement of Tregs. These Tregs decrease (inhibit) the activity of CTLs which should kill tumor cells. Chemotherapy reduces tumor cells but also Tregs population: Lymphopenia. Nevertheless, after application of the chemotherapy, CTLs expand via homeostatic proliferation. Interactions between the tumor cells and the immune system and the effects of the chemotherapy and immunotherapy have been written in terms of ordinary differential equations (ODE). A set of ODEs describes the chemotherapy and immunotherapy pharmacokinetics. Simulations have been performed (python and simulX) in order to study the sensitivity of the model’s parameters (typical values from literature). In-vitro experiments are on-going at the SMARTc wet lab (with J. Ciccolini and G. Sicard).
- Major adverse effects of Ifosfamide-Actinomycin-Vincristine (IVA) treatment for rhabdomyosarcoma are haematological toxicities such as neutropenia or thrombocytopenia. Among individual patients, the severity of these effects vary but their dynamic profiles are similar. A non-empirical adjustment of the chemotherapy dose to avoid severe levels of these toxicities could be beneficial for patients. Twenty seven patients with rhabdomyosarcoma treated with up to 9 cycles of IVA chemotherapy courses have been included. Before and during each cycle, routine multiple blood cell counts were performed allowing for a dynamic study of the haematological toxicities. We developed a machine learning analysis using gradient boosting regression techniques to forecast the IVA induced haematological toxicities. Several regressor parameters were optimized using a grid search technique. To validate models’ accuracy, we performed a cross-validation and compared predicted and observed neutrophil and platelet levels. The first four IVA cycles have been modeled as a function of neutrophil and platelet initial levels and the initial dose of Ifosfamide. The model was able to reproduce the dynamic profiles of the haematological toxicities.
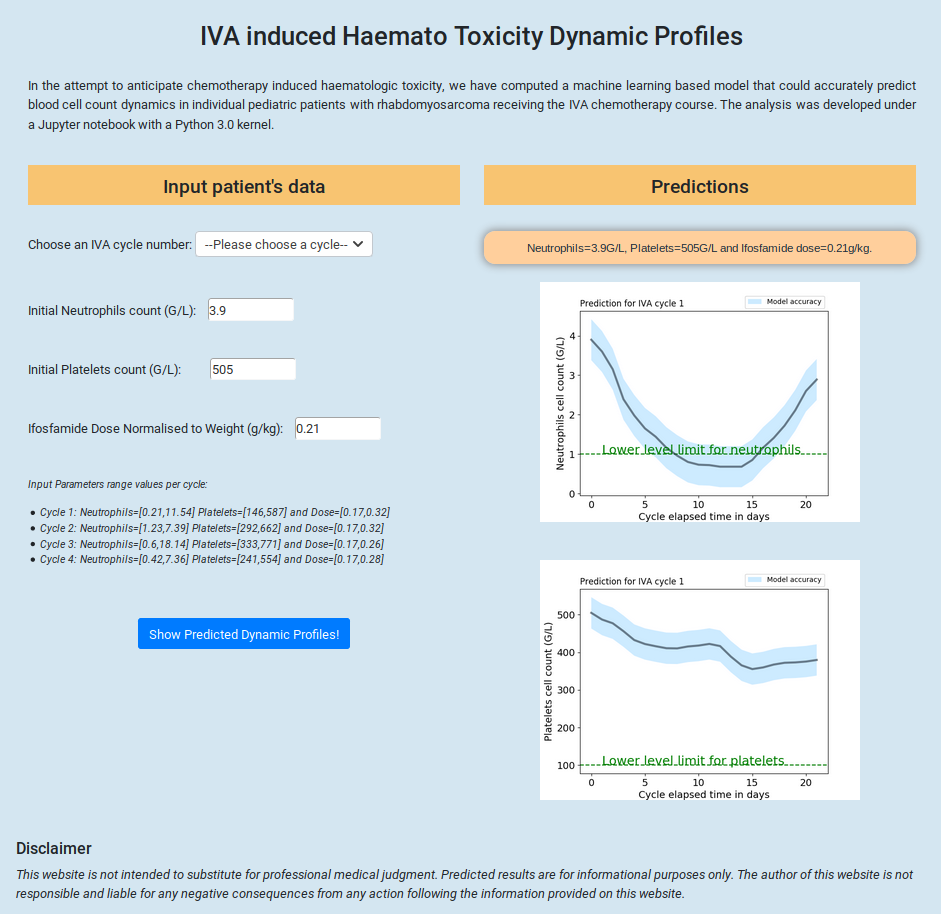
Among all cycles, the mean absolute error between predicted and observed neutrophil and platelet levels were respectively 1.1 and 79 G/L. During the first cycle, the mean absolute error on neutrophil and platelet levels were 0.53 and 39.5 G/L respectively. The dynamic profiles of haematological toxicities of patients with rhabdomyosarcoma receiving IVA chemotherapy courses were modeled using a machine learning technique. Adjusting a patient’s ifosfamide dose based on the predicted haematological toxicity levels at the end of a treatment cycle could enable a tailored follow-up during the treatment. Poster presentation at the SIOP 2019 Paediatric Oncology Congress in Lyon.
- PAIR Pancreas project in collaboration with N. Dusetti (INSERM, Campus Luminy, Marseille): Development of personalized treatments for patients with pancreatic adenocarcinoma. Pancreatic cancer is a systematically fatal disease. At a time when personalized medicine seems to be the better alternative for the treatment of most cancers, it becomes urgent to adapt the treatment to the subtypes of pancreatic adenocarcinoma and their sensitivity to available drugs (via chemograms). Combining information from chemograms and several omic studies (transcriptomics, proteomics), we envision that the identification of molecular signatures could allow to predict the clinical course and chemo-sensitivity of this disease.
2015-2016: Theranostics Simulation at the Service Hospitalier Frédéric Joliot (CEA, Orsay)
Biomedical Optics Express Vol. 8 (3) 2017 Simulation of nanoparticle-mediated near-infrared thermal therapy using GATE, V. Cuplov, F. Pain and S. Jan.
A new approach in cancer treatment is the use of gold nanoparticles for the induction of intracellular hyperthermia. At a specific wavelength of near infrared light, collective oscillation of electrons on the gold nanoparticle (NP) surface causes a phenomenon called Surface Plasmon Resonance resulting in strong extinction of light. The NP vibrates, which results in heating. In order to investigate the influence of NP properties on the spatial distribution of heat in the tumor and healthy tissues, accurate simulations are required. I extended the Geant4 based simulation code GATE to model the nanoparticle-mediated hyperthermal therapy as well as simple heat diffusion in biological tissues. This new feature of GATE combined with optical imaging allows for the simulation of a theranostic scenario in which the patient is injected with theranostic nanosystems that can simultaneously deliver therapeutic (i.e. hyperthermia therapy) and imaging agents (i.e. fluorescence imaging).
The mathematical model that best describes the thermal behavior in biological perfused tissues is the Pennes bioheat model:
The first term of the equation describes the transfer of energy between objects in contact and the second term accounts for the effects of blood perfusion in tissues. k, ρ and c are the biological tissue thermal conductivity, density and specific heat. wb is the tissue blood perfusion rate which represents the volume of fluid (blood) that passes per unit time and per tissue volume. This Equation is solved analytically via Fourier transformations and convolution theorem:
 |
The solution of the diffusion equation is equivalent to convolving the initial conditions with a Gaussian with a standard deviation σ = sqrt(2tK1). The blood perfusion term appears as an exponential function. The implementation of the heat diffusion in GATE is performed using the Insight Segmentation and Registration Toolkit (ITK). The validation of the heat diffusion in GATE with experimental data was done with a parallelepipedic phantom made of a common thermoplastic polymer, the acrylonitrile butadiene styrene (ABS – optical and thermal properties from literature) which was illuminated using a 633nm laser.
The GATE simulation and the experimental data are in good agreement as shown in Figure (a). The relative error between the data and the GATE simulation ranges from -0.86 to 5.15%. In a second part, we compared our simulation to the experimental results obtained by Hirsch et al. In their work, 5 mice were inoculated with canine transmissible venereal tumor cells. The cells were grown until the tumor reached a diameter of approximately 1 cm. NIR absorbing gold-silica nanoshells were injected into the tumor volume. After injection, tumor sites were exposed to NIR light. The GATE simulation benchmark consisted of the MOBY digital mouse phantom in which a 1 cm diameter spherical tumor was inserted under 1 mm of mouse skin tissue. The phantom tumor was infused with NIR absorbing gold-silica nanoshells. Figure (b) shows the temperature rise as a function of the depth from skin for a region of interest at the center of the tumor site for 1, 3 and 6 minutes of nanoshell-mediated near-infrared thermal therapy treatment. Thermal profiles
 |
 |
|
Figure (a): Validation between GATE and the ABS phantom. |
Figure (b): Validation between GATE and in-vivo data. |
show a good qualitative agreement between the simulation and the experimental data published by Hirsch et al. The dashed lines correspond to the simulation results with a thermal diffusivity of 0.17 mm2·s-1 and no blood perfusion. The hashed bands illustrate the effect of the blood perfusion term. The band upper limit correponds to the tumor blood perfusion rate value (0.0004 s-1) and the lower limit corresponds to the tissue blood perfusion rate value (0.004 s-1). The agreement between the GATE simulation and the mouse in vivo data is good, all the more as most of the simulation parameters (mouse absorption and scattering coefficients, thermal conductivity, specific heat and blood perfusion rates in tissue or tumor) were taken from the literature.
2013-2015: PET/CT imaging in Idiopathic Pulmonary Fibrosis at the Institute of Nuclear Medicine (UCL, London)
Physics in Medicine & Biology Vol. 63 (1) 2017 Issues in quantification of registered respiratory gated PET/CT in the lung, V. Cuplov, B. Holman, J. McClelland, M. Modat, B. Hutton and K. Thielemans.
Physics in Medicine & Biology Vol. 61 (8) 2016 The effect of respiratory induced density variations on non-TOF PET quantitation in the lung, B. Holman, V. Cuplov, B. Hutton, A. Groves, K. Thielemans.
Physics in Medicine & Biology Vol. 60 (18) 2015 Improved correction for the tissue fraction effect in lung PET/CT imaging, B. Holman, V. Cuplov, L. Millner, B. Hutton, T. Maher, A. Groves, K. Thielemans.
Idiopathic Pulmonary Fibrosis (IPF) is a fatal disease. It belongs to a large group of lung diseases known as interstitial lung diseases (ILD). Tissues deep in the lungs become scarred over time. The cause of the fibrosis is unknown. It affects middle-aged and older adults and there is no effective treatment. The prognosis is 3 to 5 years after diagnosis. The causes of death are respiratory failure, heart failure, pulmonary embolism, pneumonia and lung cancer.
PET/CT quantification in lung diseases such as IPF is affected by the lung density variability during respiration. Therefore several corrections need to be applied on the original PET image in order to take into account the lung tissue composition. Air content in the lung influences reconstructed image values. Therefore, it is important to correct for this effect called the tissue fraction effect (TFE). In a recent paper, we included the blood component in the TFE.
PET/CT quantification of lung tissue is limited by several difficulties: the lung density and local volume changes during respiration, the anatomical mismatch between PET and CT and the relative contributions of tissue, air and blood to the PET signal (the tissue fraction effect). Air Fraction Correction (AFC) has been shown to improve PET image quantification in the lungs. Methods to correct for the movement and anatomical mismatch involve respiratory gating and image registration techniques. While conventional registration methods only account for spatial mismatch, the Jacobian determinant of the deformable registration transformation field can be used to estimate local volume changes and could be used to correct (i.e. Jacobian correction, JC) the PET signal for changes in concentration due to local volume changes.

Figure showing an example of one patient CT registration with the corresponding Jacobian determinant map: gated CT in the reference gate (end-expiration) (top); gated CT in the floating gate (end-inhalation) (middle) and registered image overlaid with the Jacobian determinant map (bottom).
We investigated the relationship between variations in the lung due to respiration, specifically density, tracer concentration and local volume changes. We studied the effect of AFC and JC on PET quantitation after registration of respiratory gated PET/CT patient data. Six patients suffering from lung cancer with solitary pulmonary nodules underwent 18F-FDG PET/cineCT. The PET data were gated into six respiratory gates using displacement gating based on a Real-time Position Management signal and reconstructed with matched gated CT. The PET tracer concentration and tissue density were extracted from registered gated PET and CT images before and after corrections (AFC or JC) and compared to the values from the reference images. Across all gates and patients, the maximum relative change in PET tracer concentration before (after) AFC was found to be 16.2% (4.1%) and the maximum relative change in tissue density and PET tracer concentration before (after) JC was found to be 17.1% (5.5%) and 16.2% (6.8%) respectively.

Overall our results show that both AFC or JC largely explain the observed changes in PET tracer activity over the respiratory cycle. We also speculated that a second order effect is related to change in fluid content but this needs further investigation: a component of the lung tissue (other than parenchyma) flows in and out of the lungs over the breathing cycle, such as blood or lymph. Methods using lower dose CT acquisitions will also be investigated hoping that it will lead to extension of methods that avoid cine-CT to increase their accuracy.
2011-2013: Optical Imaging Simulation at the Service Hospitalier Frédéric Joliot (CEA, Orsay)
Applied Radiation and Isotopes Vol. 103 2015 Identifying key surface parameters for optical photon transport in GEANT4/GATE simulations, J. Nilsson, V. Cuplov, M. Isaksson.
J. Biomed. Opt. Vol. 19 (2) 2014 Extension of the GATE Monte Carlo simulation package to model bioluminescence and fluorescence imaging, V. Cuplov, I. Buvat, F. Pain and S. Jan.
In November 2011, I joined the OpenGATE collaboration (http://www.opengatecollaboration.org) working at the Service Hospitalier Frederic Joliot in Orsay in collaboration with the Laboratoire d’Imagerie et Modelisation en Neurobiologie et Cancerologie (IMNC, Orsay). GATE (Geant4 Application for Tomography Emission) is a Geant4 based toolkit for simulation of clinical and preclinical scans acquired in emission tomography, computed tomography and radiotherapy treatments. Monte-Carlo simulations are useful for the design of new imaging devices, optimization of acquisition protocols and improvement of reconstruction algorithms. In this project, I extended GATE to model optical imaging experiments such as bioluminescence and fluorescence. I integrated the relevant physics processes, added databases with optical properties of tissues and developed a module (geometry) for optical imaging. An other part of the project was to develop a hybrid version of GATE (hGATE) that will improve the computing performance by running GATE on CPU/GPU architectures and facilitating its development on the Grid. This project was done in close collaboration with physicists, biologists, computer scientists and physicians (INSERM, CEA, CNRS).
An optical imaging module for GATE
In the last decade, optical imaging has become a major modality in the preclinical setting, especially for screening cancerous models in mice. Its technique is generally non-invasive, low cost and allow for real time study of biological processes through 3D images of the light distribution emitted from the surface of small animals or superficial areas in humans. Given that optical imaging is a matter of photon transportation, I extended GATE so that it can model optical imaging experiments such as bioluminescence or fluorescence imaging.
I validated the optical photon physics by comparing the model implementation in GATE with respect to MCML (Monte-Carlo for Multi-Layered media) simulation results. Figure 1(a) shows the percentage of absorbed, transmitted and back-scattered optical photons obtained after a unidirectional source of optical photons was emitted perpendicularly to an optical phantom target of different thickness. Geant4 provides GATE with a large panel of options to simulate boundary surfaces through a set of parameters, such as possible irregularities. These are expressed by the parameter sigmaalpha which increases with the surface roughness. Figure 1(b) shows the number of absorbed, transmitted and back-scattered optical photons obtained using an optical phantom which surface was defined as perfectly smooth or rough (sigmaalpha varying from 0 to 21 degrees).
 |
 |
| Figure 1(a) | Figure 1(b) |
GATE physics of optical photon transportation in biological tissues is in agreement with the MCML simulation. The sigmaalpha parameter does not influence much the measurement of the transmittance, absorbance and reflectance in biological tissues. The Wave Length Shifting process from Geant4 has been added to GATE in order to simulate fluorescence. Figure~\ref{fluorescence} compares the fluorescent photon wavelength spectrum obtained from experimental measurements in our laboratory and with GATE. This work has been presented at the IEEE-MIC conference in Anaheim, California in November 2012.
 |
 |
| Figure 1(a) | Figure 1(b) |
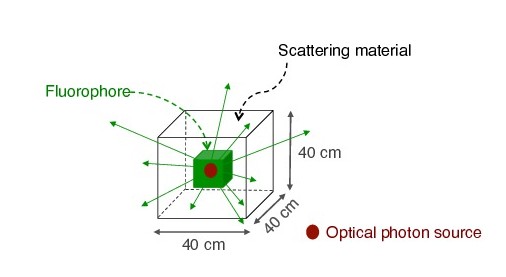
As a proof of concept of optical imaging simulations using GATE, we performed the experiences shown below. In case of the bioluminescence experiment (Figure 2), the center of a voxelized source (which represents the tumor) of optical photons was positioned under the inner layer of the phantom. Each voxel was assigned a specific optical photon flux. In case of the fluorescence experiment (Figure 3), we used a voxelized tumor and assigned the Rhodamine B fluorophore to each voxel of the tumor and positioned it under the inner layer of the phantom. The fluorophore was excited by an external beam light source emitting optical photons towards the tumor. The optical imaging system was composed of an array made of pixels, an electronic board and an angular aperture that limits the range of angles over which the optical system can accept light.
 |
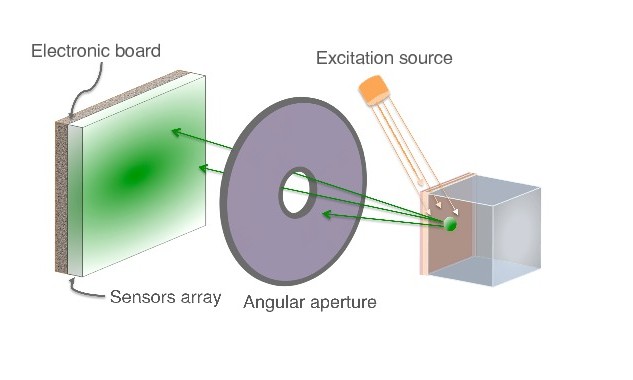 |
| Figure 2 | Figure 3 |
We simulated different scenarii for the phantom’s external layers that were made of water, epidermis or hypodermis. In each of these simulations we tracked 10^{10} photons and obtained the projections (on the detecting surface plane, i.e. sensors array) of the bioluminescent or fluorescent light emitted from the surface of the phantom. Figure 4 shows the 2D image and profile of the bioluminescence light emitted from the surface of the phantom. The width of the detected bioluminescent light distribution increases with the phantom complexity due to scattering of light in biological tissues.
 |
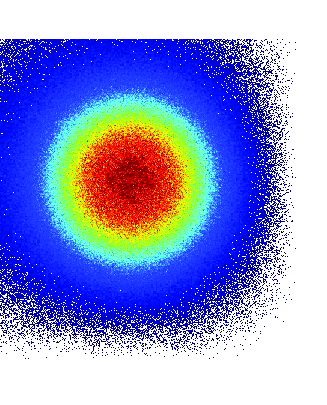 |
 |
| Figure 4 |
A bioluminescence experiment was also simulated using GATE and a complex voxelized phantom (MOBY) that contained the following organs: brain, lungs filled with air, kidney, bladder filled with water and all other organs described as soft tissue (hypodermis).
 |
 |
| 2D images of the bioluminescence light at the surface of the complex voxelized MOBY phantom. |
GATE is capable of simulating optical tomography by rotating (i.e orbiting) the optical system or by duplicating it along a ring around the patient. The resulting GATE tomographic projections can be combined into an inversion scheme to estimate the sources (e.g. fluorophore dimension and location) that produced the observable image (i.e. fluorescent light distribution). This work has been presented at the IEEE-MIC conference in 2012 (IEEE Medical Imaging Conference record (NSS/MIC 2012). V. Cuplov, I. Buvat, M. Mesradi, F. Pain and S. Jan – Optical Imaging Simulation using GATE) and published in the Journal of Biomedical Optics in 2014 (Extension of the GATE Monte Carlo simulation package to model bioluminescence and fuorescence imaging, V. Cuplov, I. Buvat, F. Pain and S. Jan. J. Biomed. Opt. 19 (2), 026004 (2014)).
Implementation of the optical imaging module for GPU architecture
Monte-Carlo simulations being computationally demanding, we run them on multi-processors. Graphics processing units or GPUs now offer a solution to significantly reduce execution times. I developed a proof of concept code specific to the main GATE imaging applications that runs on GPU architecture. This code is currently being integrated within GATE where the voxelized phantom tracking is performed on GPU and the detection on CPU. I implemented the optical photon Mie scattering physics process following the Henyey-Greenstein approximation which describes the angular distribution of the light scattered by small particles. This was done with the CUDA (Compute Unified Device Architecture) computing platform created by NVIDIA.
The validation set-up that was used to compare our results was made of a voxelised phantom of 10x10x10 voxels; four of them were assigned with an isotropic optical photon flux. Figure 5 shows the comparison between the Mie scattering GPU code and the GATE-CPU. The acceleration factor due to the photon tracking on GPU was found to be 100 for an optical imaging simulation (Mie scattering only).
 |
 |
 |
| Figure 5:CPU/GPU |
This work has been presented at the IEEE-MIC conference in 2012 (IEEE Medical Imaging Conference record, 2012. J. Bert, H. Perez-Ponce, S. Jan, Z. El Bitar, P. Gueth, V. Cuplov, H. Chekatt, D. Benoit, D. Sarrut, Y. Boursier, D. Brasse, I. Buvat, C. Morel et D. Visvikis – Hybrid GATE: A GPU/CPU implementation for imaging and therapy applications). Recently, I implemented the Fresnel Reflectance calculation which gives the fraction of the incident power that is reflected from the boundary between two media (i.e. biological tissues). Using the reflectance value, a Monte-Carlo technique is used to decide whether the optical photon is reflected or refracted and the Snell-Decartes law is applied. It describes the relationship between the angles of incidence and refraction. The validation of the current version of the Fresnel processes which deals only with the geometry of reflection and refraction is in progress. Further work aims in a general version of the Fresnel processes with the implementation of the Normal vector to the surface pointing out of previous volume and into current volume such as it is done in the Geant4 navigator and the reflection and transmission coeffecients as a function of the optical photon polarization.